All matter consists of atoms. Atoms are the building blocks of everything and when arranged in a particular way will form the principal classes of matter which are mixtures and pure substances. Learning and visualizing the structure of an atom is the first step in understanding the elements that you see in the periodic table. Elements are substances with a single type of atom (i.e. carbon atoms when aggregated can form diamond or graphite, pure gold is gold atoms, a titanium metal sheet consists of only titanium atoms).
Atomic Structure
In the 1890s, it was discovered that atoms can be broken down into smaller particles: subatomic particles (refer to Chapter 2 of your textbook). These particles are protons, neutrons, and electrons. The nucleus, located at the center of the atom, consists of protons and neutrons. Protons are positively charged particles and are equal to one atomic mass unit (amu).
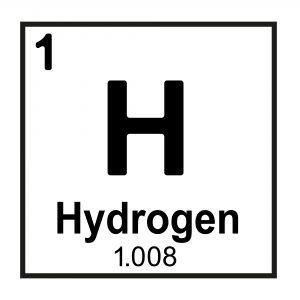
Elements differ by their number of protons (e.g. Hydrogen has 1 proton, Helium has 2 protons, and so on) which is denoted by their atomic number (also denoted “Z’; the number above the element symbol in the periodic table, typically on the left-hand side see Figure 1).
To learn more about the characteristics of subatomic particles view the thinglink image below.
The image below depicts the structure of an atom. Use your mouse to hover over the picture for the star hotspot icons to appear. When you hover over the star icons, information regarding the subatomic particles will appear.
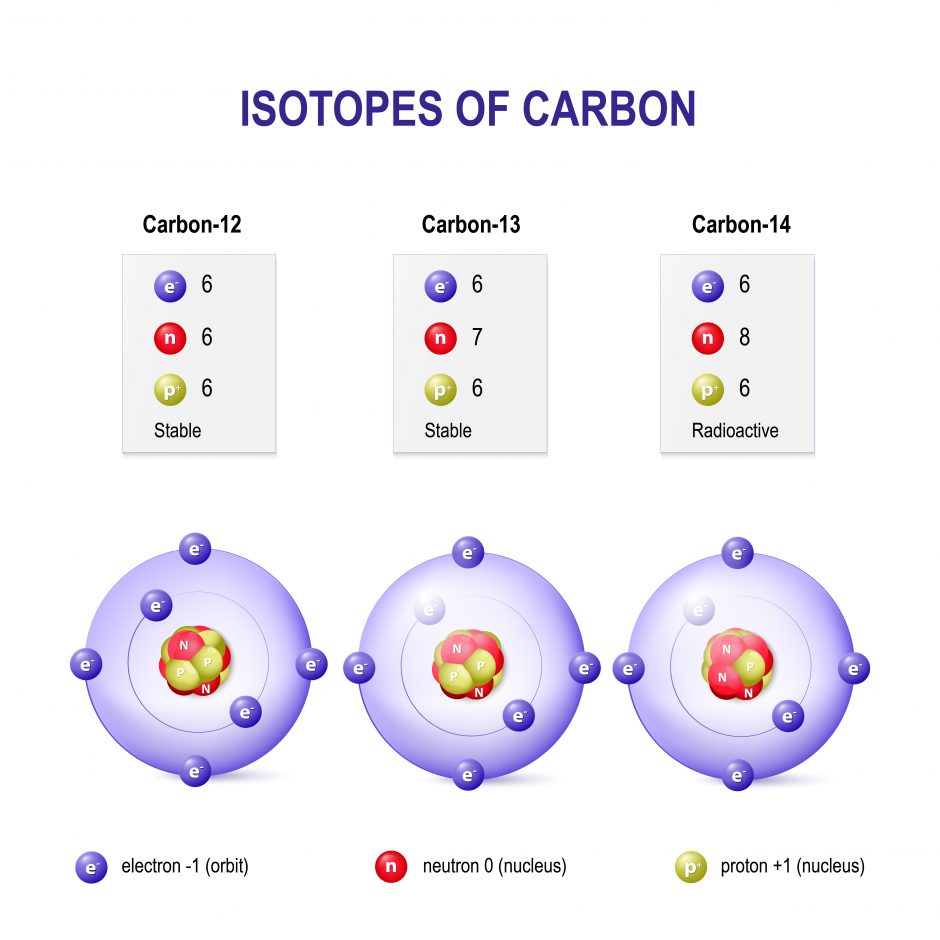
The nucleus (central part of the atom) consists of protons and neutrons. Protons are positively charged and are equal to one atomic mass unit (amu). Elements differ by their number of protons (e.g. Hydrogen has 1 proton, Helium has 2 protons). The neutron is also located in the nucleus. The neutron does not have a charge and is, therefore, a neutral particle. It has a mass of slightly over 1 amu. Differences in the number of neutrons produce isotopes of the same element (see Figure 2). Isotopes occur for a lot of the elements, which are all present in nature but in different amounts (a.k.a. natural abundance). When writing about isotopes, we write the mass of the isotope after the name: carbon-12, carbon-13, or carbon-14.
Moving outside of the nucleus is where you will find the electron that is a negatively charged particle. Due to electrons moving near the speed of light, their precise location cannot be identified so we name the space they occupy “orbitals”. You will learn later about orbital configurations.
The following interactive shows how the electrons move around the nucleus. Change the speed of the electrons by changing the delay between finding electrons from short (faster) to long (slower).
In the next section (Video Resources), a video is presented that will show you a 3D model of how an atom looks. Notice how electrons are modeled as a dense cloud around the nucleus.
If you think about elements, all of the elements in the periodic table are “happy” or relatively stable. This is due to these atoms have just the right number of subatomic particles, either in the nucleus or the electron orbitals. If you add or take away one of these particles, this leads to instability. As you progress to the simulation, you will be able to identify what makes an atom stable or unstable. Atoms may also become charged (where the atom becomes slightly positively or negatively charged), which are called ions.
Proceed to Video Resources