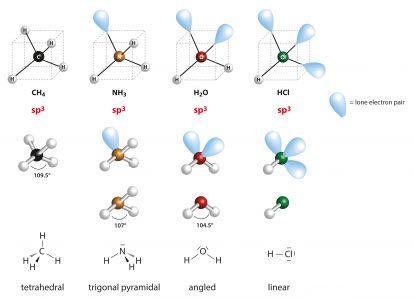
The molecular geometry is important to understand because it affects how the molecule behaves and interacts with other molecules. The valence shell electron pair repulsion (VSEPR) theory is how the geometry of a molecule is determined. The shapes that are possible are tetrahedral, trigonal planar, trigonal pyramidal, bent, and linear. To determine the shape of the molecule, you must look at the central atom. Unbonded electrons around the center are not accounted for in the geometry, however, they are important because they determine the geometry. Unbonded electrons around atoms that are not the central atom have little effect on the geometry. The figure below gives some examples of molecular geometry along with lone electron pairs. The hybridization is also listed.
Check out the interactive below to see the different molecular shapes and electron geometries.
When we look at a water molecule, you can see that the central atom, oxygen, is dictating the shape. Oxygen has 8 electrons which means there are four pair arranged in tetrahedral shape.
Proceed to Video Resources